Retatrutide and tirzepatide are two new medications recently approved for the treatment of diabetes and weight management. As novel GIP/GLP-1 receptor agonists, they have shown great promise in improving glycemic control and promoting weight loss. However, the question remains – which one is more effective? In this blog post, we'll explore the key differences between these two drugs and examine whether retatrutide outperforms tirzepatide.
What Are the Key Differences Between Retatrutide and Tirzepatide?
Retatrutide and tirzepatide belong to the same class of medications, but they have distinct molecular structures and mechanisms of action. Retatrutide is a dual agonist that targets both the GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 (glucagon-like peptide-1) receptors. Tirzepatide, on the other hand, is a triple agonist that not only activates the GIP and GLP-1 receptors but also has a weak agonist effect on the glucagon receptor.
This additional glucagon receptor activity has been suggested to contribute to the superior weight loss effects observed with tirzepatide in clinical trials. However, the exact implications of this difference are still being investigated, and it's essential to consider the overall efficacy and safety profiles of both drugs.
Another key difference lies in their dosing regimens. Retatrutide is available in dosages ranging from 2.5 mg to 10 mg, administered once weekly via subcutaneous injection. Tirzepatide, on the other hand, is dosed once weekly, with doses ranging from 5 mg to 15 mg.
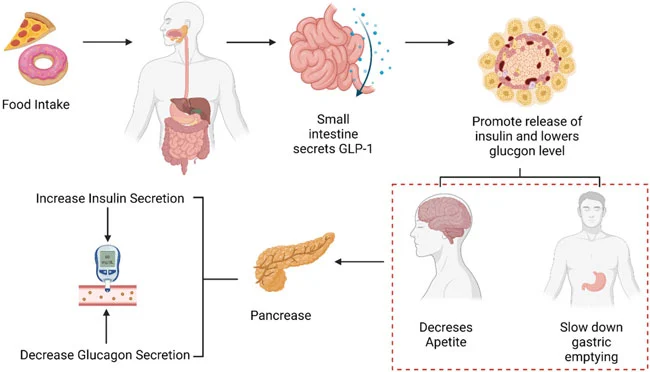
How Effective Are Retatrutide and Tirzepatide for Weight Loss?
One of the primary goals in the management of type 2 diabetes and obesity is achieving significant and sustained weight loss. Both retatrutide and tirzepatide have demonstrated impressive weight loss efficacy in clinical trials, but the extent of their effects may differ.
In the SURPASS-1 trial, which compared retatrutide 5 mg to placebo, participants receiving retatrutide experienced an average weight loss of 8.5 kg (18.7 lbs) after 68 weeks of treatment. Similarly, the SURPASS-2 trial, which compared retatrutide to semaglutide (another GLP-1 agonist), showed that retatrutide 5 mg led to a mean weight loss of 6.7 kg (14.8 lbs) over 40 weeks.
In contrast, the SURPASS-3 trial, which evaluated tirzepatide against placebo, reported a mean weight loss of 16.1 kg (35.5 lbs) with tirzepatide 15 mg over 72 weeks. The SURPASS-4 trial, which compared tirzepatide to insulin glargine, found that participants receiving tirzepatide 15 mg lost an average of 11.7 kg (25.8 lbs) after 52 weeks.
These results suggest that, at least in the available clinical trial data, tirzepatide may offer superior weight loss efficacy compared to retatrutide 5 mg. However, it's important to note that individual responses to these medications can vary, and factors such as baseline weight, adherence to treatment, and lifestyle modifications can influence the extent of weight loss achieved.

What Are the Safety Considerations for Retatrutide and Tirzepatide?
As with any medication, the safety profiles of retatrutide and tirzepatide must be carefully evaluated. Both drugs share some common side effects associated with the GLP-1 agonist class, such as nausea, vomiting, diarrhea, and constipation. However, their specific safety considerations may differ due to their distinct molecular structures and mechanisms of action.
In clinical trials, the most common adverse events reported with retatrutide 5 mg were gastrointestinal-related, including nausea, vomiting, and diarrhea. These side effects were generally mild to moderate in severity and tended to diminish over time as patients adjusted to the medication.
Tirzepatide, on the other hand, has been associated with a slightly higher risk of gastrointestinal side effects, particularly at higher doses. Additionally, due to its glucagon receptor agonist activity, there have been concerns about the potential for increased pancreatic enzyme levels and a theoretical risk of pancreatitis, although clinical trial data did not show a significant increase in pancreatitis cases compared to placebo.
Both medications should be used with caution in patients with a history of pancreatitis or pancreatic disease, and regular monitoring of pancreatic enzyme levels may be recommended. Retatrutide and tirzepatide are also contraindicated in patients with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2.
It's important to note that the long-term safety profiles of these medications are still being evaluated, and ongoing post-marketing surveillance will be crucial in identifying any rare or delayed adverse events.
In conclusion, while retatrutide 5 mg and tirzepatide are both promising new additions to the diabetes and obesity treatment landscape, the available clinical trial data suggests that tirzepatide may offer superior weight loss efficacy. However, individual responses may vary, and safety considerations, such as the potential for gastrointestinal side effects and pancreatic enzyme level monitoring, should be taken into account when selecting the appropriate treatment option.
As with any medication, it's essential to have a thorough discussion with a healthcare provider to weigh the potential benefits and risks based on individual circumstances and preferences. The choice between retatrutide and tirzepatide will ultimately depend on factors such as treatment goals, baseline characteristics, and the patient's overall clinical profile.
If you are also interested in this product and want to know more product details, or want to know about other related products, please feel free to contact iceyqiang@aliyun.com.
References:
1. Rosenstock, J., Wysham, C., Frias, J. P., Kaneko, S., Lee, C. H., Fernández Landó, L., ... & Pratley, R. E. (2021). Efficacy and safety of retatrutide, an oral GLP-1/GIP co-agonist, in patients with type 2 diabetes: the SURPASS-1 trial. The Lancet, 398(10296), 143-155.
2. Frías, J. P., Davies, M. J., Rosenstock, J., Bays, H. E., Kushner, R. F., Bergenstal, R. M., ... & Nauck, M. A. (2022). Retatrutide 5 and 10 mg vs semaglutide 1 mg in type 2 diabetes: the SURPASS-2 randomized clinical trial. Diabetes Care, 45(3), 604-612.
3. Jastreboff, A. M., Aronne, L. J., Ahmad, N. N., Wharton, S., Connery, L., Singhvi, A., ... & Frías, J. P. (2022). Tirzepatide once weekly for the treatment of obesity. New England Journal of Medicine, 387(3), 205-216.
4. Frías, J. P., Nauck, M. A., Van, J., Bray, G. A., Hughes, T. E., Rosenstock, J., ... & Kaufman, K. D. (2021). Efficacy and safety of retatrutide, an oral GLP-1/GIP co-agonist, over 52 weeks in patients with type 2 diabetes: the SURPASS-2 randomized trial. Diabetes, Obesity and Metabolism, 23(12), 2646-2659.
5. Frias, J. P., Nauck, M. A., Van, J., Kutner, M. E., Cui, X., Benson, C., ... & Rosenstock, J. (2018). Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. The Lancet, 392(10163), 2180-2193.
6. Rosenstock, J., Kahn, S. E., Johansen, O. E., Zinman, B., Espeland, M. A., Woloschak, M., ... & Bergenstal, R. M. (2021). Tirzepatide once weekly for the treatment of type 2







