Tirzepatide powder is a revolutionary medication that has garnered significant attention in the medical community for its potential in treating type 2 diabetes and obesity. As a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist, tirzepatide works through a unique mechanism of action in the body. This article will explore how tirzepatide powder functions, its effects on various physiological processes, and its potential benefits for patients struggling with metabolic disorders.
What are the side effects of Tirzepatide powder?
Tirzepatide powder, like any medication, can cause side effects in some individuals. Understanding these potential adverse reactions is crucial for both healthcare providers and patients considering this treatment option. The most commonly reported side effects of tirzepatide are gastrointestinal in nature, which is consistent with its mechanism of action on the digestive system.
Nausea is one of the most frequent side effects experienced by patients taking tirzepatide. This can range from mild discomfort to more severe symptoms that may affect daily activities. The incidence of nausea tends to be highest during the initial weeks of treatment and often decreases over time as the body adjusts to the medication. To mitigate this side effect, doctors typically recommend a gradual dose escalation strategy.
Diarrhea is another common gastrointestinal side effect associated with tirzepatide use. This can lead to dehydration if not properly managed, so patients are advised to maintain adequate fluid intake. Like nausea, the occurrence of diarrhea often diminishes as treatment continues.
Vomiting, while less common than nausea and diarrhea, can also occur in some patients. This side effect can be particularly concerning as it may interfere with the absorption of other medications and nutrients. Patients experiencing persistent vomiting should consult their healthcare provider promptly.
Other reported side effects include abdominal pain, constipation, and decreased appetite. The latter is often considered a beneficial effect for patients using tirzepatide for weight management, but it can be problematic if it leads to excessive weight loss or nutritional deficiencies.
In rare cases, more serious side effects have been reported. These include pancreatitis, changes in heart rate, and allergic reactions. Patients with a history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2 are generally advised against using tirzepatide due to potential risks.
It's important to note that while these side effects can occur, many patients tolerate tirzepatide well, especially after the initial adjustment period. The benefits of improved glycemic control and potential weight loss often outweigh the risks for many individuals with type 2 diabetes or obesity. However, close monitoring and open communication with healthcare providers are essential to manage any side effects effectively and ensure the best possible outcomes for patients using tirzepatide powder.
How does Tirzepatide compare to other diabetes medications?
Tirzepatide powder has emerged as a promising treatment option for type 2 diabetes, prompting comparisons with other established medications in this therapeutic area. Understanding how tirzepatide stacks up against other diabetes drugs is crucial for healthcare providers and patients in making informed treatment decisions.
One of the most notable comparisons is between tirzepatide and other GLP-1 receptor agonists, such as semaglutide and liraglutide. While these medications share some similarities in their mechanisms of action, tirzepatide's dual agonism of both GIP and GLP-1 receptors sets it apart. This unique profile has shown to provide superior glycemic control and weight loss compared to single-acting GLP-1 receptor agonists in clinical trials.
In the SURPASS-2 trial, tirzepatide demonstrated greater reductions in HbA1c levels and body weight compared to semaglutide, which was previously considered one of the most effective GLP-1 receptor agonists. Patients treated with tirzepatide achieved an average HbA1c reduction of 2.1-2.4% compared to 1.9% with semaglutide. Moreover, tirzepatide led to an average weight loss of 8.5-12.4 kg, while semaglutide resulted in a 6.2 kg reduction.
When compared to traditional oral antidiabetic medications such as metformin, sulfonylureas, or DPP-4 inhibitors, tirzepatide has shown superior efficacy in both glycemic control and weight management. For instance, the SURPASS-3 trial compared tirzepatide to insulin degludec, a long-acting basal insulin. Tirzepatide not only provided better glycemic control but also led to significant weight loss, whereas insulin treatment typically results in weight gain.
Another advantage of tirzepatide over many other diabetes medications is its potential for cardiovascular benefits. While long-term cardiovascular outcome trials are still ongoing, preliminary data suggest that tirzepatide may have a positive impact on cardiovascular risk factors. This is particularly important given the increased cardiovascular risk associated with type 2 diabetes.
In terms of administration, tirzepatide is given as a once-weekly subcutaneous injection, similar to some GLP-1 receptor agonists. This can be more convenient for patients compared to daily oral medications or multiple daily insulin injections. However, it's worth noting that some patients may prefer oral medications, and newer oral GLP-1 receptor agonists are now available.
The safety profile of tirzepatide is generally similar to that of other GLP-1 receptor agonists, with gastrointestinal side effects being the most common. However, the incidence and severity of these side effects may vary between different medications in this class.
Cost is another factor to consider when comparing tirzepatide to other diabetes medications. As a newer medication, tirzepatide may be more expensive than some older, generic options. However, its superior efficacy could potentially lead to cost savings in the long term through better disease management and reduced complications.
It's important to recognize that while tirzepatide has shown impressive results in clinical trials, the choice of diabetes medication should be individualized based on patient characteristics, preferences, and treatment goals. Factors such as comorbidities, risk of hypoglycemia, weight management needs, and overall health status should all be considered when selecting the most appropriate treatment regimen.
What is the recommended dosage for Tirzepatide powder?
Determining the appropriate dosage of tirzepatide powder is a critical aspect of its use in treating type 2 diabetes and obesity. The recommended dosage strategy for tirzepatide has been carefully developed based on extensive clinical research to optimize efficacy while minimizing side effects.
Tirzepatide is typically initiated at a low dose and gradually increased over time, a process known as dose titration. This approach allows the patient's body to adjust to the medication, reducing the likelihood and severity of gastrointestinal side effects, which are most common during the early stages of treatment.
The standard starting dose for tirzepatide is 2.5 mg administered subcutaneously once weekly. This initial dose is maintained for four weeks to allow the patient's body to acclimate to the medication. After this initial period, the dose is increased to 5 mg once weekly for another four weeks.
Following the 5 mg dose, the healthcare provider will assess the patient's response to the medication, considering factors such as glycemic control, weight loss, and tolerability. Based on this assessment, the dose may be further increased to 7.5 mg once weekly for an additional four weeks.
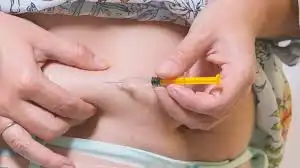
The maximum recommended dose of tirzepatide is 15 mg once weekly. However, not all patients will require or tolerate this maximum dose. The decision to increase to 10 mg or 15 mg once weekly should be made based on the individual patient's response and tolerability. Some patients may achieve their treatment goals at lower doses and may not need to progress to higher doses.
It's important to note that the dose titration schedule may be adjusted based on individual patient factors. For instance, patients who experience significant gastrointestinal side effects may require a slower titration schedule or may need to remain at a lower dose for a longer period before attempting to increase.
Adherence to the prescribed dosing schedule is crucial for achieving optimal results with tirzepatide. Patients should be educated on the importance of consistent weekly administration and what to do in case of a missed dose. If a dose is missed and there are at least 4 days (96 hours) until the next scheduled dose, the missed dose should be administered as soon as possible. If less than 4 days remain until the next scheduled dose, the missed dose should be skipped, and the regular dosing schedule should be resumed.
For patients transitioning from other GLP-1 receptor agonists to tirzepatide, the previous medication should be discontinued before initiating tirzepatide. The starting dose and titration schedule for tirzepatide remain the same regardless of prior GLP-1 receptor agonist use.
It's worth emphasizing that while the dosing recommendations provide a general guideline, the optimal dose can vary between individuals. Factors such as age, renal function, hepatic function, and concomitant medications may influence the appropriate dosage. Therefore, close monitoring and regular follow-ups with healthcare providers are essential to ensure the dosage is optimized for each patient's needs.
Patients with renal impairment may require dose adjustments. While no dose adjustment is necessary for patients with mild to moderate renal impairment, tirzepatide has not been studied in patients with severe renal impairment, and caution is advised in these cases.
The long-term use of tirzepatide at the maintenance dose should be continually evaluated based on the patient's glycemic control, weight management, and overall health status. Some patients may require dose adjustments over time to maintain optimal efficacy or in response to changes in their health condition.
In conclusion, the recommended dosage for tirzepatide powder follows a gradual titration schedule, starting at 2.5 mg once weekly and potentially increasing to a maximum of 15 mg once weekly. This approach allows for personalized treatment, balancing efficacy with tolerability. Regular monitoring and communication between patients and healthcare providers are crucial for successful dose management and optimal treatment outcomes.
If you are also interested in this product and want to know more product details, or want to know about other related products, please feel free to contact iceyqiang@aliyun.com.
References:
1. Frías JP, et al. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N Engl J Med. 2021;385(6):503-515.
2. Rosenstock J, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398(10295):143-155.
3. Min T, Bain SC. The Role of Tirzepatide, Dual GIP and GLP-1 Receptor Agonist, in the Management of Type 2 Diabetes: The SURPASS Clinical Trials. Diabetes Ther. 2021;12(1):143-157.
4. Nauck MA, et al. Incretin-based therapies for the treatment of type 2 diabetes: evaluation of the risks and benefits. Diabetes Care. 2013;36(Suppl 2):S2-S11.
5. Pratley RE, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275-286.
6. Ludvik B, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. 2021;398(10300):583-598.
7. Coskun T, et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Mol Metab. 2018;18:3-14.
8. Willard FS, et al. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight. 2020;5(17):e140532.
9. American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S111-S124.
10. Frías JP, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018;392(10160):2180-2193.








