Pregabalin powder, a potent medication primarily used to treat neuropathic pain, epilepsy, and generalized anxiety disorder, has gained significant attention in the medical community due to its unique mechanism of action within the nervous system. This article delves into the intricate workings of pregabalin powder, exploring its effects on neurotransmitters, calcium channels, and overall neuronal activity to provide a comprehensive understanding of its therapeutic benefits.
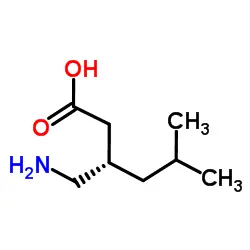
What are the main uses of pregabalin powder?
Pregabalin powder, marketed under various brand names including Lyrica, has proven to be a versatile medication with a wide range of clinical applications. Its primary uses encompass three main areas: neuropathic pain management, epilepsy treatment, and anxiety disorder therapy.
In the realm of neuropathic pain management, pregabalin has shown remarkable efficacy in alleviating discomfort associated with conditions such as diabetic neuropathy, postherpetic neuralgia, and fibromyalgia. Its ability to modulate pain signals within the nervous system makes it a valuable tool for patients struggling with chronic pain that is often resistant to conventional analgesics.
For epilepsy treatment, pregabalin serves as an adjunctive therapy for partial seizures in adults. Its anticonvulsant properties help reduce the frequency and intensity of seizures, offering improved quality of life for individuals living with epilepsy. The medication's ability to stabilize neuronal excitability contributes to its effectiveness in seizure control.
In the context of anxiety disorders, pregabalin has demonstrated significant benefits in managing generalized anxiety disorder (GAD). Its anxiolytic effects stem from its influence on neurotransmitter systems involved in anxiety regulation. By modulating these systems, pregabalin helps reduce excessive worry, restlessness, and other symptoms associated with GAD.
Beyond these primary indications, pregabalin has also shown promise in off-label uses, including the treatment of alcohol and benzodiazepine withdrawal, restless legs syndrome, and certain types of chronic pain not typically associated with neuropathy. However, it's important to note that these off-label uses should only be considered under the guidance of a healthcare professional.
The versatility of pregabalin powder in addressing multiple neurological and psychiatric conditions underscores its importance in modern medicine. Its unique mechanism of action, which we will explore in more detail in the following sections, allows it to target multiple aspects of neural function, making it a valuable tool in the treatment of complex neurological disorders.
How does pregabalin affect neurotransmitter release?
Pregabalin's influence on neurotransmitter release is a crucial aspect of its mechanism of action within the nervous system. While it doesn't directly bind to GABA receptors or alter GABA uptake, its effects on neurotransmitter release are significant and multifaceted.
The primary mechanism by which pregabalin affects neurotransmitter release is through its binding to the α2δ subunit of voltage-gated calcium channels. These channels play a critical role in the release of neurotransmitters at synapses throughout the nervous system. By binding to the α2δ subunit, pregabalin reduces the influx of calcium into neurons, which in turn leads to a decrease in the release of several excitatory neurotransmitters.
The neurotransmitters most affected by pregabalin's action include glutamate, norepinephrine, and substance P. Glutamate, the primary excitatory neurotransmitter in the central nervous system, is particularly important in the context of pain signaling and neuronal excitability. By reducing glutamate release, pregabalin helps to dampen excessive neuronal firing associated with neuropathic pain and seizures.
Norepinephrine, another neurotransmitter affected by pregabalin, plays a crucial role in the body's stress response and is implicated in anxiety disorders. The reduction in norepinephrine release contributes to pregabalin's anxiolytic effects, helping to alleviate symptoms of generalized anxiety disorder.
Substance P, a neuropeptide involved in pain signaling, is also affected by pregabalin's action on calcium channels. The decreased release of substance P contributes to the medication's analgesic properties, particularly in the context of neuropathic pain.
It's important to note that while Pregabalin Powder reduces the release of these excitatory neurotransmitters, it does not completely block their release. Instead, it modulates their release, helping to restore a more balanced state of neuronal activity. This modulation, rather than complete inhibition, is part of what makes pregabalin effective while minimizing potential side effects associated with complete neurotransmitter blockade.
Moreover, pregabalin's effects on neurotransmitter release are not uniform across all neural circuits. The medication appears to have a more pronounced effect on hyperexcited neurons, such as those involved in chronic pain conditions or epilepsy, while having less impact on normal neuronal function. This selective action contributes to its therapeutic efficacy and favorable side effect profile compared to some other medications used for similar conditions.
The complex interplay between pregabalin's binding to calcium channels and its subsequent effects on neurotransmitter release underlies its diverse therapeutic applications. By modulating the release of key excitatory neurotransmitters, pregabalin helps to restore balance in neural circuits disrupted by conditions such as neuropathic pain, epilepsy, and anxiety disorders.
What role do calcium channels play in pregabalin's mechanism of action?
Calcium channels play a pivotal role in pregabalin's mechanism of action, serving as the primary target through which the medication exerts its therapeutic effects. Understanding the interaction between pregabalin and these channels is crucial to comprehending how the drug influences neural function and provides relief for various neurological and psychiatric conditions.
Voltage-gated calcium channels are integral membrane proteins that allow the influx of calcium ions into cells in response to changes in membrane potential. In neurons, these channels are particularly important for several processes, including neurotransmitter release, gene expression, and the regulation of neuronal excitability. The α2δ subunit, to which pregabalin binds, is an auxiliary protein associated with these channels and plays a crucial role in their function and trafficking to the cell membrane.
Pregabalin's high-affinity binding to the α2δ subunit, specifically the α2δ-1 and α2δ-2 isoforms, results in a reduction of calcium influx into neurons. This reduction occurs through two main mechanisms:
1. Decreased channel trafficking: Pregabalin powder interferes with the normal trafficking of calcium channels to the cell surface. By binding to the α2δ subunit, it reduces the number of functional calcium channels available at synaptic terminals.
2. Altered channel kinetics: The binding of pregabalin to the α2δ subunit can also affect the opening and closing kinetics of the calcium channels, further reducing calcium influx.
The consequences of this reduced calcium influx are far-reaching and underlie many of pregabalin's therapeutic effects:
1. Reduced neurotransmitter release: As discussed in the previous section, the decreased calcium influx leads to a reduction in the release of excitatory neurotransmitters like glutamate, norepinephrine, and substance P.
2. Decreased neuronal excitability: By limiting calcium entry, pregabalin helps to dampen the overall excitability of neurons, which is particularly beneficial in conditions characterized by hyperexcitability, such as epilepsy and neuropathic pain.
3. Modulation of synaptic plasticity: Calcium plays a crucial role in synaptic plasticity, the ability of synapses to strengthen or weaken over time. By modulating calcium influx, pregabalin may influence long-term changes in neural circuits, potentially contributing to its sustained therapeutic effects.
4. Neuroprotection: Excessive calcium influx can be neurotoxic. By limiting calcium entry, pregabalin may offer some neuroprotective effects, although this aspect requires further research.
It's important to note that pregabalin's effects on calcium channels are state-dependent, meaning they are more pronounced in hyperexcited neurons. This selectivity contributes to its efficacy in treating conditions characterized by neuronal hyperexcitability while minimizing effects on normal neuronal function.
The role of calcium channels in pregabalin's mechanism of action extends beyond just neurotransmitter release. The modulation of these channels influences various aspects of neuronal function, from signal transmission to long-term changes in neural circuits. This broad impact on neuronal physiology explains pregabalin's efficacy across a range of neurological and psychiatric conditions, from neuropathic pain to anxiety disorders.
Moreover, the specific targeting of the α2δ subunit by pregabalin represents a unique approach in pharmacotherapy. Unlike medications that directly target neurotransmitter systems, pregabalin's modulation of calcium channels allows for a more nuanced regulation of neural activity. This approach often results in a favorable balance between efficacy and side effects, making pregabalin a valuable option in the treatment of various neurological disorders.
In conclusion, the intricate interplay between pregabalin and voltage-gated calcium channels, mediated through the α2δ subunit, forms the cornerstone of its therapeutic action. By modulating these channels, Pregabalin Powder influences multiple aspects of neuronal function, providing relief for a diverse array of neurological and psychiatric conditions. As research continues, our understanding of this mechanism may lead to even more refined approaches to treating disorders of the nervous system.
If you are also interested in this product and want to know more product details, or want to know about other related products, please feel free to contact iceyqiang@aliyun.com.
References
1. Taylor, C. P., Angelotti, T., & Fauman, E. (2007). Pharmacology and mechanism of action of pregabalin: the calcium channel α2–δ (alpha2–delta) subunit as a target for antiepileptic drug discovery. Epilepsy Research, 73(2), 137-150.
2. Dooley, D. J., Taylor, C. P., Donevan, S., & Feltner, D. (2007). Ca2+ channel α2δ ligands: novel modulators of neurotransmission. Trends in Pharmacological Sciences, 28(2), 75-82.
3. Bauer, C. S., Nieto-Rostro, M., Rahman, W., Tran-Van-Minh, A., Ferron, L., Douglas, L., ... & Dolphin, A. C. (2009). The increased trafficking of the calcium channel subunit α2δ-1 to presynaptic terminals in neuropathic pain is inhibited by the α2δ ligand pregabalin. Journal of Neuroscience, 29(13), 4076-4088.
4. Patel, R., & Dickenson, A. H. (2016). Mechanisms of the gabapentinoids and α2δ-1 calcium channel subunit in neuropathic pain. Pharmacology Research & Perspectives, 4(2), e00205.
5. Gajraj, N. M. (2007). Pregabalin: its pharmacology and use in pain management. Anesthesia & Analgesia, 105(6), 1805-1815.
6. Tzellos, T. G., Toulis, K. A., Goulis, D. G., Papazisis, G., Zampeli, V. A., Vakfari, A., & Kouvelas, D. (2010). Gabapentin and pregabalin in the treatment of fibromyalgia: a systematic review and a meta-analysis. Journal of Clinical Pharmacy and Therapeutics, 35(6), 639-656.
7. Boschen, M. J. (2011). A meta-analysis of the efficacy of pregabalin in the treatment of generalized anxiety disorder. Canadian Journal of Psychiatry, 56(9), 558-566.
8. Fink, K., Dooley, D. J., Meder, W. P., Suman-Chauhan, N., Duffy, S., Clusmann, H., & Göthert, M. (2002). Inhibition of neuronal Ca2+ influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology, 42(2), 229-236.
9. Micheva, K. D., Taylor, C. P., & Smith, S. J. (2006). Pregabalin reduces the release of synaptic vesicles from cultured hippocampal neurons. Molecular Pharmacology, 70(2), 467-476.
10. Verma, V., Singh, N., & Singh Jaggi, A. (2014). Pregabalin in neuropathic pain: evidences and possible mechanisms. Current Neuropharmacology, 12(1), 44-56.








