What are the main applications of magnesium ethoxide in catalysis?
Magnesium ethoxide has found its place in numerous catalytic applications, showcasing its versatility and efficiency in various chemical processes. One of the primary areas where magnesium ethoxide excels as a catalyst is in organic synthesis reactions. It has proven to be particularly effective in promoting carbon-carbon bond formation, such as in aldol condensations and Michael additions. These reactions are crucial in the synthesis of complex organic molecules, including pharmaceuticals and fine chemicals.
In the field of polymerization, magnesium ethoxide has demonstrated its catalytic prowess. It serves as an excellent initiator for the ring-opening polymerization of cyclic esters, such as lactones and lactides. This application is particularly valuable in the production of biodegradable polymers, which are gaining importance in various industries due to their environmentally friendly nature. The use of magnesium ethoxide as a catalyst in these polymerization reactions offers advantages such as high catalytic activity, good control over molecular weight, and the ability to produce polymers with narrow molecular weight distributions.
Another significant application of magnesium ethoxide in catalysis is in transesterification reactions. This process is crucial in the production of biodiesel, where vegetable oils or animal fats are converted into fatty acid methyl esters. Magnesium ethoxide has shown promise as an effective heterogeneous catalyst for these reactions, offering benefits such as easy separation from the reaction mixture and potential reusability. The use of magnesium ethoxide in biodiesel production aligns with the growing interest in sustainable and renewable energy sources.
Furthermore, magnesium ethoxide has found applications in the synthesis of metal oxides and mixed metal oxides. When used as a precursor in sol-gel processes, it can facilitate the formation of high-purity metal oxide materials with controlled compositions and structures. These materials have applications in areas such as catalysis, sensors, and advanced ceramics. The ability of magnesium ethoxide to form homogeneous solutions and undergo controlled hydrolysis makes it an attractive choice for these synthesis routes.
How does magnesium ethoxide compare to other metal alkoxides in catalytic activity?
When comparing magnesium ethoxide to other metal alkoxides in terms of catalytic activity, several factors come into play. The unique properties of magnesium ethoxide, such as its moderate Lewis acidity and high basicity, contribute to its catalytic performance in various reactions. However, it's essential to consider how it stacks up against other commonly used metal alkoxides, such as those of aluminum, titanium, and zirconium.
In terms of Lewis acidity, magnesium ethoxide generally exhibits milder acidity compared to alkoxides of aluminum or titanium. This characteristic can be advantageous in reactions where strong Lewis acids might lead to unwanted side reactions or product degradation. The moderate Lewis acidity of magnesium ethoxide makes it particularly suitable for reactions that require a balance between activation of substrates and selectivity towards desired products.
On the other hand, the high basicity of magnesium ethoxide sets it apart from many other metal alkoxides. This property makes it an excellent choice for base-catalyzed reactions, such as transesterifications and aldol condensations. In these reactions, magnesium ethoxide often outperforms other metal alkoxides, providing higher yields and selectivities under milder reaction conditions.
When it comes to polymerization reactions, particularly the ring-opening polymerization of cyclic esters, magnesium ethoxide has shown comparable or sometimes superior performance to other metal alkoxides. Its ability to initiate polymerization while maintaining good control over molecular weight and polydispersity makes it a competitive choice in this field. However, titanium and zirconium alkoxides are also widely used in polymerization catalysis and may be preferred in certain specific applications due to their unique electronic and steric properties.
In the context of sol-gel processes and metal oxide synthesis, magnesium ethoxide offers some distinct advantages. Its relatively low cost and ease of handling make it an attractive option for large-scale applications. Additionally, the magnesium oxide formed from magnesium ethoxide precursors often exhibits high surface area and porosity, which can be beneficial for catalytic applications. However, other metal alkoxides, particularly those of transition metals like titanium and zirconium, are often preferred when specific electronic or optical properties are desired in the final metal oxide product.
It's worth noting that the catalytic activity of metal alkoxides, including magnesium ethoxide, can be significantly influenced by factors such as the presence of co-catalysts, reaction conditions, and the specific requirements of the target reaction. Therefore, while general comparisons can be made, the choice between magnesium ethoxide and other metal alkoxides often depends on the particular application and desired outcome.
What are the advantages and limitations of using magnesium ethoxide as a catalyst?
Magnesium ethoxide offers several advantages as a catalyst, making it an attractive choice for various chemical processes. One of its primary benefits is its relatively low toxicity compared to many other metal-based catalysts. This characteristic makes magnesium ethoxide more environmentally friendly and safer to handle in industrial settings. Additionally, magnesium is an abundant element, which translates to lower costs and improved sustainability in catalyst production.
Another significant advantage of magnesium ethoxide is its versatility. As discussed earlier, it can catalyze a wide range of reactions, from organic syntheses to polymerizations and transesterifications. This versatility allows for its use in multiple applications within the same facility, potentially reducing the need for diverse catalyst inventories. Furthermore, magnesium ethoxide often exhibits high catalytic activity under mild conditions, which can lead to energy savings and reduced wear on equipment in industrial processes.
The basic nature of magnesium ethoxide makes it particularly effective in reactions that benefit from base catalysis. This property, combined with its moderate Lewis acidity, allows it to promote both acid- and base-catalyzed reactions, sometimes simultaneously. This dual functionality can be advantageous in complex reaction systems or in cascade reactions where multiple steps are carried out in a single pot.
In terms of product quality, magnesium ethoxide often promotes high selectivity in many reactions. This selectivity can lead to higher yields of desired products and reduced formation of by-products, which is crucial in fine chemical and pharmaceutical synthesis. Additionally, in polymerization reactions, magnesium ethoxide can offer good control over molecular weight and polydispersity, resulting in polymers with more consistent properties.
However, like any catalyst, magnesium ethoxide also has its limitations. One of the main challenges is its sensitivity to moisture and air. Magnesium ethoxide can readily hydrolyze or oxidize when exposed to these elements, which can reduce its catalytic activity or lead to unwanted side reactions. This sensitivity necessitates careful handling and storage procedures, which can add complexity and cost to industrial processes.
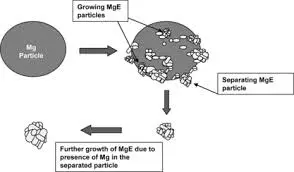
Another limitation is the potential for magnesium incorporation into the final product, especially in metal oxide synthesis or certain organic reactions. While this may be desirable in some applications, it can be problematic when high-purity products are required. In such cases, additional purification steps may be necessary, increasing the overall process complexity and cost.
The moderate Lewis acidity of magnesium ethoxide, while advantageous in many situations, can be a limitation in reactions that require strong Lewis acid catalysis. In these cases, other metal alkoxides or traditional Lewis acids may be more effective. Similarly, while magnesium ethoxide is a good base catalyst, there are situations where stronger bases might be preferred, limiting its applicability in certain strongly base-catalyzed reactions.
Finally, the solubility of magnesium ethoxide can be both an advantage and a limitation. While it is soluble in many organic solvents, which facilitates homogeneous catalysis, its solubility can make catalyst recovery and recycling more challenging compared to heterogeneous catalysts. This aspect is particularly important in large-scale industrial applications where catalyst recovery can significantly impact process economics.
In conclusion, magnesium ethoxide presents a compelling option as a catalyst in various chemical processes. Its versatility, relatively low toxicity, and ability to promote high selectivity make it an attractive choice for many applications. However, its sensitivity to moisture and air, potential for product contamination, and limitations in strongly acidic or basic reactions must be carefully considered when evaluating its use as a catalyst. As with any catalyst selection, the specific requirements of the reaction and process conditions should guide the decision to use magnesium ethoxide.
If you are also interested in this product and want to know more product details, or want to know about other related products, please feel free to contact iceyqiang@aliyun.com.